
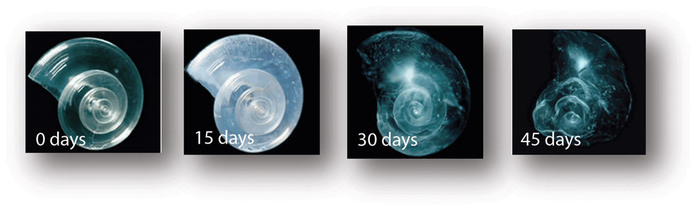
When carbon dioxide (CO2) ( produced by burning fossil fuels, industrial pollution and other human interventions) is absorbed by seawater, chemical reactions occur that reduce seawater pH, and literally destroy vital minerals like calcium carbonate. These chemical reactions are termed "ocean acidification" or "OA" for short. Calcium carbonate minerals are the building blocks for the skeletons and shells of many marine organisms. In areas where most of the aquatic life congregates in the ocean, the seawater is supersaturated with calcium carbonate minerals. This means there are abundant building blocks for calcifying organisms to build their skeletons and shells. However, continued ocean acidification is causing many parts of the ocean to become undersaturated with these minerals, which affects the ability of many organisms to produce and maintain their shells.
Since the beginning of the Industrial Revolution, the pH of surface ocean waters has fallen by 0.1 pH units. Since the pH scale, like the Richter scale, is logarithmic, this change represents approximately a 30 percent increase in acidity. Future predictions indicate that the oceans will continue to absorb carbon dioxide and become even more acidic. Estimates of future carbon dioxide levels, based on business as usual emission scenarios, indicate that by the end of this century the surface waters of the ocean could be nearly 150 percent more acidic, resulting in a pH that the oceans haven’t experienced for more than 20 million years.
The Biological Impacts
Ocean acidification is expected to impact ocean species to varying degrees. Photosynthetic algae and seagrasses may benefit from higher CO2 conditions in the ocean, as they require CO2 to live just like plants on land. On the other hand, studies have shown that a more acidic environment has a dramatic effect on calcifying species, including oysters, clams, sea urchins, shallow water corals, deep sea corals, and calcareous plankton and many others. Each year, more shelled species become at risk, and to clarify, an immediate effect on humans would be the loss of shrimp, crabs and lobsters. When shelled organisms are at risk, the entire food chain is at risk. Other, larger forms of sea-life live from consuming these species. When those larger species can't find food, they disappear and the even larger fish that relied on those creatures for food also become extinct and so on up the food chain until it reaches humans.Today, more than a billion people worldwide rely on food from the ocean as their primary source of protein. In the future, the reliance on the oceans as a source of food for the world will become greater.
Many jobs and economies in the U.S. and all around the world depend on the fish and shellfish in our oceans. Many species of fish have already been driven to the brink of extinction by over-fishing and depleting supplies. A solution to the acidification of the oceans is by simply reducing carbon-dioxide in our atmosphere. The same solution for global warming.

No comments:
Post a Comment